India Reaches 400 Million COVID-19 Vaccine Doses on July 3, 2021
2021 · New Delhi, India
India administers over 400 million doses of COVID-19 vaccines, making it the fastest country to achieve this milestone.
December 14, 2020
The United States initiated its COVID-19 vaccination campaign with the first doses of the Pfizer-BioNTech vaccine administered to healthcare workers and high-priority groups.
Long Island, United States | Pfizer Inc., BioNTech SE, United States government
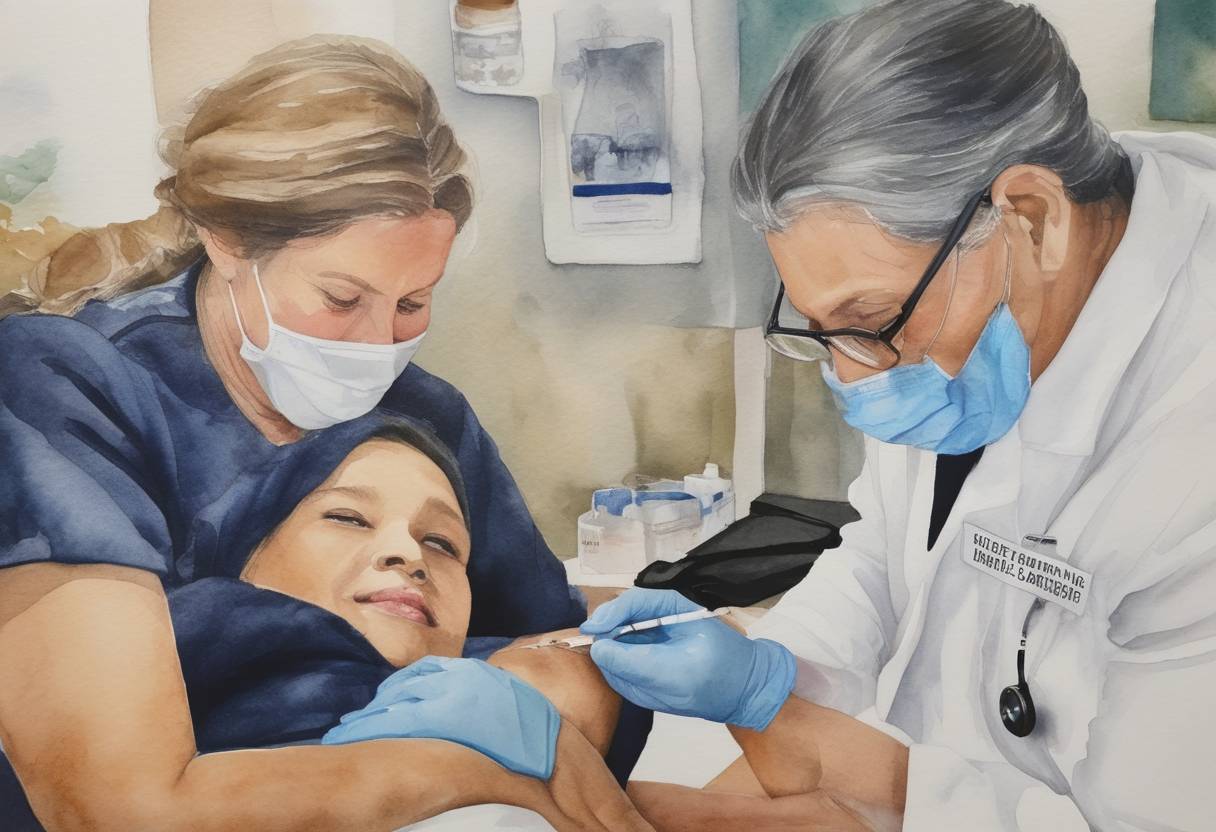
On December 14, 2020, the United States began its COVID-19 vaccination campaign with the administration of the first doses of the Pfizer-BioNTech vaccine. This marked a significant milestone in the fight against the COVID-19 pandemic, which had profoundly impacted the nation and the world throughout 2020.
The emergence of COVID-19 in late 2019 led to a global pandemic, with the U.S. experiencing significant outbreaks, resulting in high infection rates and mortality. The urgent need for a vaccine prompted an unprecedented rapid development and approval process for several vaccine candidates.
The Pfizer-BioNTech vaccine, known scientifically as BNT162b2, was developed through a collaboration between the American pharmaceutical company Pfizer and the German biotechnology company BioNTech. Utilizing mRNA technology, the vaccine demonstrated a high efficacy rate of approximately 95% in preventing COVID-19 in clinical trials. On December 11, 2020, the U.S. Food and Drug Administration (FDA) issued an Emergency Use Authorization (EUA) for the vaccine, paving the way for its distribution.
The first vaccinations under the new campaign took place in several locations across the United States. High-priority groups, primarily healthcare workers and residents of long-term care facilities, were prioritized to receive the vaccine, in alignment with guidelines from the Centers for Disease Control and Prevention (CDC).
The initiation of the vaccination campaign was a pivotal moment in the United States’ response to the COVID-19 pandemic. It represented a scientific breakthrough and showcased the potential of mRNA technology as a tool in combating infectious diseases.
The distribution and administration phases required meticulous planning and logistics, considering the Pfizer-BioNTech vaccine’s storage requirements at ultra-cold temperatures. It also marked the beginning of broader efforts to vaccinate the general public, with subsequent phases planning to include additional vaccines such as Moderna’s mRNA-1273, which received EUA later in December 2020.
The December 14, 2020, launch of the COVID-19 vaccination campaign was a testament to international collaboration, innovation, and the resilience of healthcare systems in the face of a global crisis.
Source: www.nytimes.com